Mihoko Kai Lab Research
|
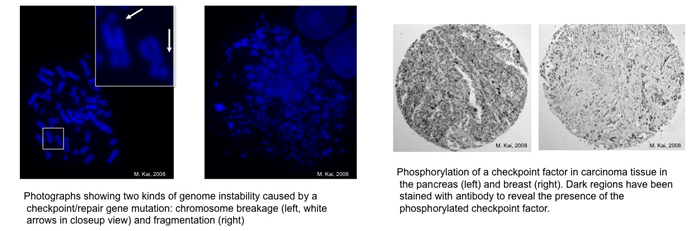
The main focus of Dr. Kai’s laboratory is to understand how genome stability is maintained in cells. Nature has evolved two highly conserved processes in order to cope with chromosomal perturbations: DNA repair and the cell cycle checkpoint. Mutations in repair and checkpoint genes have been implicated in cancer-prone syndromes in human cells. Improper coordination and control of these two mechanisms can lead to the accumulation of mutations and genome instability. But while the repair and checkpoint pathways have been separately well studied, understanding of the relationship between them has lagged. We are presently investigating four aspects of this relationship, employing a two-pronged research strategy that involves both yeast and human cells. This approach allows us to obtain fast and powerful results at the basic level, and also apply them rapidly to mammalian cell studies that are directly relevant to human diseases. |
|
Please contact Dr. Kai directly if you are interested in joining us on one or more of these projects.
1. Mechanisms of DNA polymerase switch in response to replication stress
DNA
replication is a perilous stage during the cell cycle, and one that is
particularly vulnerable to DNA damage and perturbation. The DNA replication
machinery must liaise with repair and checkpoint machineries in order to
maintain genomic integrity.
Genetic data obtained in fission yeast has led us to a current working hypothesis
that one of the replication factors is involved in the signal transduction
pathway. The signal from replication fork stall needs to be transmitted to
checkpoint and repair pathways. This replication factor could be the key for
the first signal. Alternatively, the signal from the checkpoint might go to
this replication factor to activate a repair pathway. This replication factor
is also known to interact directly with PCNA, which is required for both
replication and repair. This interaction might be important for the signal
transduction to activate a repair pathway. In order to understand the signal
transduction mechanism, we use genetic and biochemical analysis in both fission
yeast and human cells. These studies will answer longstanding questions: how
does “polymerase switch” occur? How are repair pathways activated when
replication fork is stalled?
2. Function of Werner Syndrome (WRN) Helicase SUMOylation
Werner Syndrome is an autosomal recessive disorder characterized by predisposition to cancer and premature aging, which is caused by mutations in the WRN helicase gene. Recent studies indicate that WRN helicase plays significant role not only in DNA repair and telomere maintenance, but also in DNA replication. It has also been shown that WRN helicase becomes sumoylated. However, the function of this sumoylation and the function of WRN helicase during replication both remain obscure. Biochemical and genetic analyses indicate that WRN sumoylation might be linked to the checkpoint and repair pathways. The project here will be to elucidate the nature and function of this link.
3. Function of cell cycle checkpoint factors at telomeres
Upstream checkpoint factors are involved in maintenance of telomeres in yeast. Deletion of Rad3 and Tel1 (ATR and ATM homologues) induces acute telomere loss. However, mutations in other checkpoint factors, such as Rad17, Rad1, Rad9 and Hus1, cause shortening of telomeres, while mutations in checkpoint kinases, Cds1 and Chk1, produce no telomere defect. It appears that the regulation of telomere length does not require the classic signal transduction pathway of the checkpoint. The upstream checkpoint factors may regulate other telomere-maintenance proteins. Preliminary results indicate that some modification of the checkpoint factors is involved in this process. We would like to better understand what this modification does, and why.
4. Identification of novel tumor suppressors and oncogenes
What factors accelerate the accumulation of small mutations and gross chromosomal changes that leads to malignancy? The transcriptional level of the mutagenic polymerase k is up-regulated in response to damage. This up-regulation requires checkpoint activation in fission yeast. Now, polymerase k is up-regulated in lung cancer cells, and some checkpoint factors are also up-regulated in cancer cells. Exactly why, when and how do these factors become up-regulated? What are the consequences? Checkpoint activation should cause cell cycle arrest or cell death. In cancer cells, however, neither occurs if the up-regulated proteins are active. To resolve the apparent paradox, we are conducting RNAi screening to identify the negative and positive regulators. This project would be particularly suitable for somebody with experience in siRNA screening techniques.
|
Recent Publications |
● Kai. M., Kanji Furuya, Francesca Paderi, Antony M. Carr, and Teresa S.F. Wang. Rad3-dependent phosphorylation of the checkpoint clamp regulates repair pathway choice. Nature Cell Biology, 2007; 9:691-697.
● Kai, M., Boddy, M.N., Russell, P. and Wang, T.S. Replication checkpoint kinase Cds1 regulates Mus81 to preserve genome integrity during replication stress. Genes & Development. 2005; 19: 919-932
● Kai, M. and Wang, T.S. Checkpoint response to replication stalling: inducing tolerance and preventing mutagenesis (review). Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2003 532: 59-73
● Kai, M. and Wang, T. S.-F. Checkpoint activation regulates mutagenesis translesion synthesis. Genes & Development. 2003 17 (1): 64-76.
● Kai, M., Tanaka, H. and Wang, T.S. Fission Yeast Rad17 Associates with Chromatin in Response to Aberrant Genomic Structures. Mol. Cell. Biol. 2001 May 15; 21(10):3289-3301.
